September 2023
Nature
Li X, Wu F, Günther S, Looso M, Kuenne C, Zhang T, Wiesnet M, Klatt S, Zukunft S, Fleming I, Poschet G, Wietelmann A, Atzberger A, Potente M, Yuan X, Braun T.
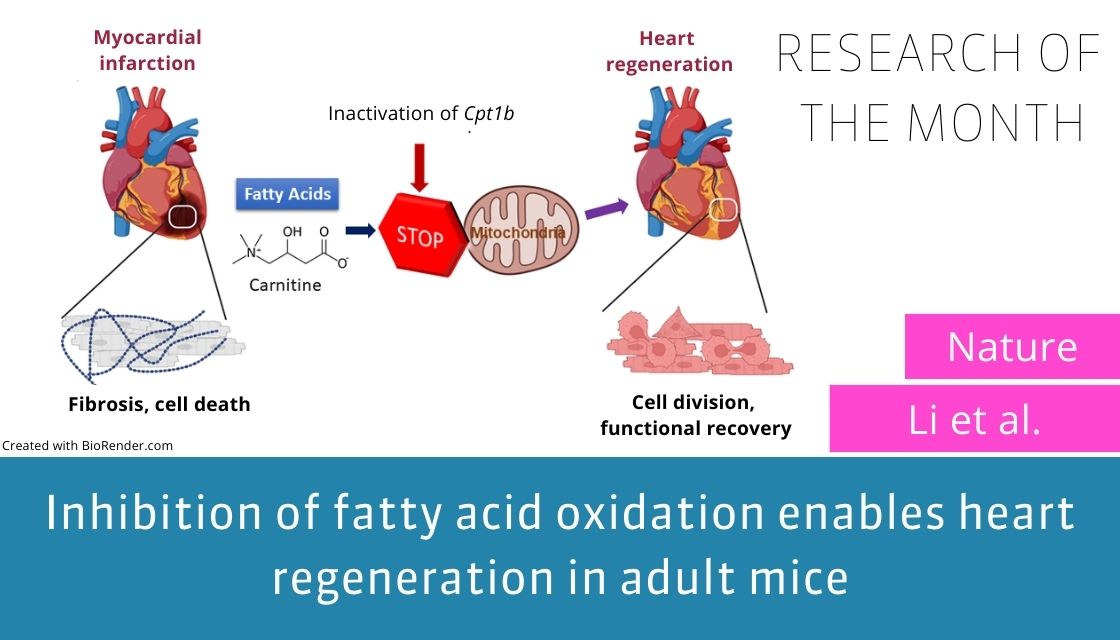
Mammalian hearts lose the ability for efficient regeneration shortly after birth. Therefore, severe injuries or diseases of the heart often lead to permanent damage, eventually resulting in heart failure with fatal consequences. Loss of cardiomyocyte (CM) proliferative capacity after birth is the main reason for ineffective regeneration in adult mammalian hearts. Arrest of proliferation during CM maturation is accompanied by fundamental changes the energy metabolism, among others. In the fetal and early neonatal stages, when CMs still proliferate, CMs mainly utilize glycolysis for ATP production. Shortly after birth, CMs shift the energy metabolism to fatty acid oxidation and commit to permanent cell cycle withdrawal.
In the recent study, published in Nature, Li et al. discovered that inhibition of fatty acids oxidation by inactivation of Cpt1b in CMs reverts CMs to a less mature state, and enables CM proliferation and heart regeneration after ischemic reperfusion. Remarkably, the number of CMs in the heart nearly doubles after inactivation of Cpt1b, which is critical for the uptake of fatty acids into mitochondria. Metabolome analysis revealed a nearly 20-times increase of the Citrate Cycle metabolite alpha-ketoglutarate (aKG) in CMs, due to by enhanced production and reduced consumption within the Citrate Cycle. Further experiments indicated that aKG stimulates the activity of Jmjc-domain containing histone demethylases, leading to reduced presence of the histone modification H3K4me3, which is required for normal expression of cardiac identity genes. Li et al, also identified the histone demethylase KDM5B as the main driver of H3K4me3 demethylation. Overexpression of Kdm5b recapitulates parts of the Cpt1b deficiency phenotype in CMs, including stimulation of CM proliferation, whereas knockdown of Kdm5b attenuates proliferation of neonatal CMs, induced by treatment with cell-permeable aKG. These data indicate that KDM5 servers as a nuclear sensor for changes in the energy metabolism of CMs. In summary, the study disclosed an intricate interplay between energy metabolism and the epigenome in CMs, which can be exploited to stimulate hearts regeneration as a potential new therapy for the treatment of heart disease.