February 2024
Nat Commun.
DNMT3A clonal hematopoiesis-driver mutations induce cardiac fibrosis by paracrine activation of fibroblasts
Shumliakivska M, Luxán G, Hemmerling I, Scheller M, Li X, Müller-Tidow C, Schuhmacher B, Sun Z, Dendorfer A, Debes A, Glaser SF, Muhly-Reinholz M, Kirschbaum K, Hoffmann J, Nagel E, Puntmann VO, Cremer S, Leuschner F, Abplanalp WT, John D, Zeiher AM, Dimmeler S.
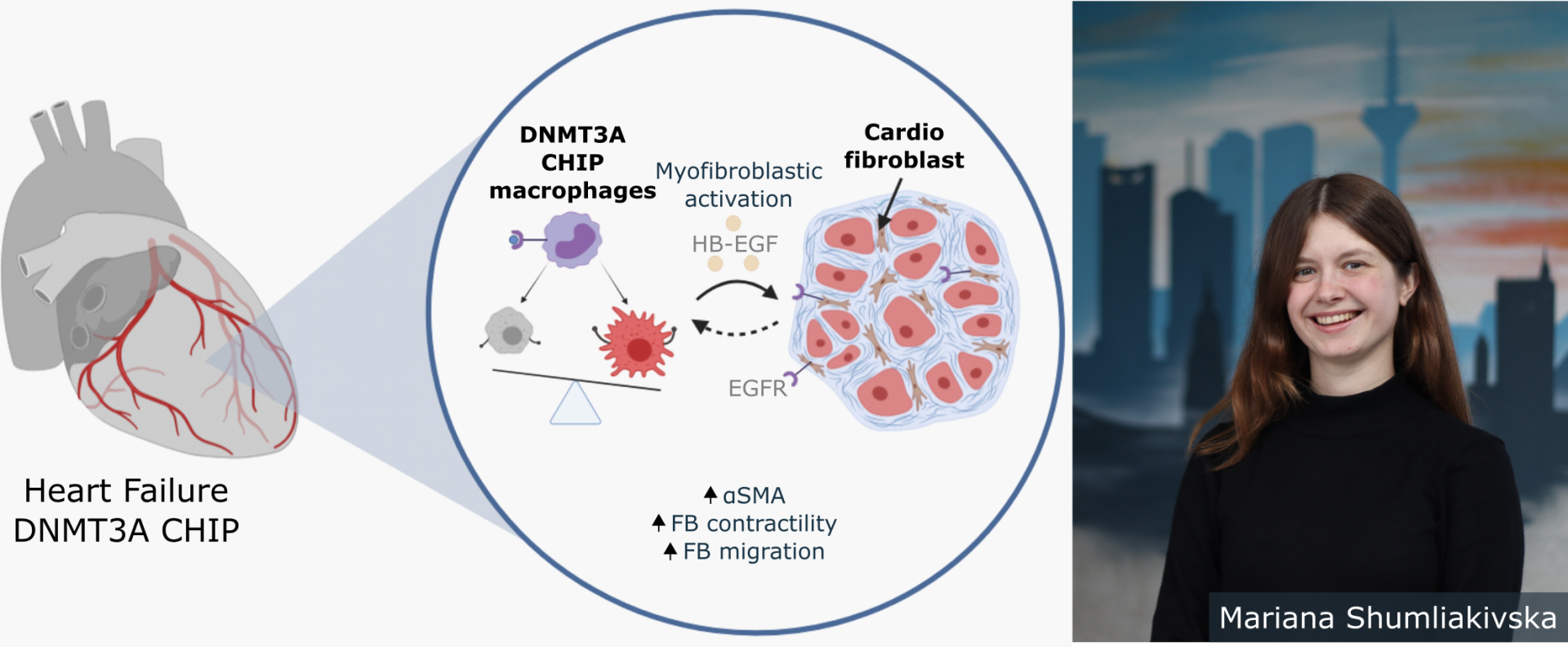
We are proud of CPI junior researcher Mariana Shumliakivska, who has achieved a fine success in heart failure research. In her recent study, she found, that specific genetic modification of blood cells that happen at old age contribute to cardiac fibrosis. How this mechanism is involved in the development of heart failure was previously unclear. These processes cause the heart to stiffen and perform less efficiently. Understanding those underlying genetic modifications could improve the development of new therapies in the future.
Here are the details:
Somatic mutations of hemapoietic stem cells (blood cell precursors) often affect epigenetic regulators, like the DNA methlytransferase 3A (DNMT3A). Inactivation of DNMT3A in monocytes increases monocyte interaction with cardiac fibroblasts, inducing cardiac fibrosis. Hereby an increased release of heparin-binding epidermal growth factor-like growth factor (HB-EGF) in activating cardiac fibroblasts plays a crucial role. This study lays the groundwork for potential anti-fibrotic strategies.