August 2023
Science
Wagner JUG, Tombor LS, Malacarne PF, Kettenhausen LM, Panthel J, Kujundzic H, Manickam N, Schmitz K, Cipca M, Stilz KA, Fischer A, Muhly-Reinholz M, Abplanalp WT, John D, Mohanta SK, Weber C, Habenicht AJR, Buchmann GK, Angendohr S, Amin E, Scherschel K, Klöcker N, Kelm M, Schüttler D, Clauss S, Günther S, Boettger T, Braun T, Bär C, Pham MD, Krishnan J, Hille S, Müller OJ, Bozoglu T, Kupatt C, Nardini E, Osmanagic-Myers S, Meyer C, Zeiher AM, Brandes RP, Luxán G, Dimmeler S.
As modern life conditions and medical care have remarkably improved, the current society increasingly age. However, while a prolonged life-span is an undoubtable triumph, age-associated pathologies such as cardiovascular diseases increase. Hence understanding cardiovascular ageing is one key to improve not only the life- but also the health-span.
In this regard, the endothelium has gained special attention. It was shown in the bone-marrow, the liver and the lungs, that endothelial cells maintain tissue homeostasis and may exert reparative functions in a paracrine manner. However, the role of endothelial cells in cardiac aging remains less clear.
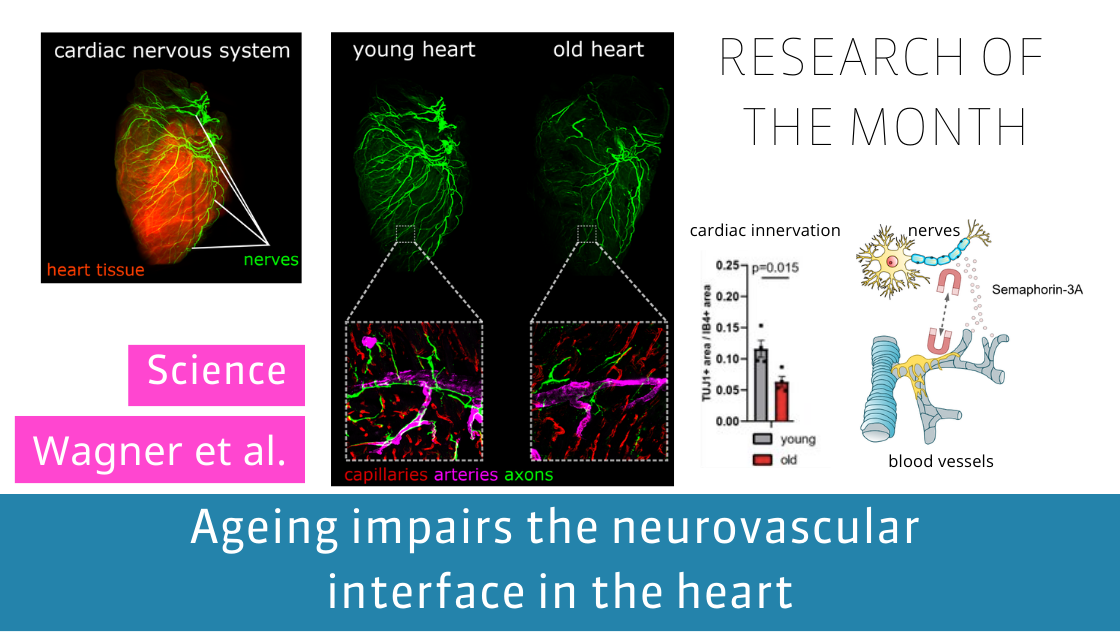
In our current study, we have discovered a significant upregulation in the expression and release of the axon-repelling factor Semaphorin-3A by endothelial cells, resulting in the regression of nervous fibers in the aging left ventricle of the heart. Remarkably, this upregulation of Semaphorin-3A was found to be triggered by the downregulation of miR-145, a microRNA that diminishes in the aged heart. Intriguingly, this process appears to be closely tied to cellular senescence.
Through a comprehensive examination of mice throughout their adult lifespan, we observed a sudden decline in cardiac innervation occurring at 16 months of age, concurrent with the development of cellular senescence within the heart tissue. Moreover, the expression levels of miR-145 and Semaphorin-3A were closely associated with the senescence process. The regression of nervous fibers had a direct impact on heart rate variability in aged mice, a phenomenon that was successfully reversed by administering two senolytic drugs, dasatinib and quercetin, to eliminate cellular senescence. Additionally, also the nervous regression and Semaphorin-3A expression were reversed in these hearts.
In summary, our findings shed light on the relationship between endothelial senescence and the neuro-vascular interface during cardiac aging. Understanding and targeting these mechanisms hold promise for novel therapeutic approaches to tackle cardiovascular diseases.
More on this topic in the media:
- Press: 2023 October Editorial on “Age-induced senescence impairs the neurovascular interface in the heart” (nature reviews cardiology)
- Press: 2023 September 14 – Stefanie Dimmeler, Julian Wagner: Wenn das Herz die Nerven verliert (Herzmedizin)
- Press: 2023 August 25 – Stefanie Dimmeler, Julian Wagner: Alternde Nerven bringen das Herz aus dem Takt (Frankfurter Allgemeine Zeitung)
- Press: 2023 August 25 – Stefanie Dimmeler, Julian Wagner: Wenn das Herz die Nerven verliert (DeutschesGesundheitsPortal)