September 2020
EMBO J
Linc‐MYH configures INO80 to regulate muscle stem cell numbers and skeletal muscle hypertrophy
Abstract:
Chromatin remodeling complexes have functions in transcriptional regulation and chromosome maintenance, but it is mostly unknown how the function of these normally ubiquitous complexes is specified in the cellular context. Here, we describe that the evolutionary conserved long non‐coding RNA linc‐MYH regulates the composition of the INO80 chromatin remodeler complex in muscle stem cells and prevents interaction with WDR5 and the transcription factor YY1. Linc‐MYH acts as a selective molecular switch in trans that governs the pro‐proliferative function of the ubiquitous INO80 complex but does not affect its role in maintaining genomic stability. The molecular switch is essential for restricting generation of quiescent MuSCs and proliferation of myoblasts in homeostasis and regeneration. Since linc‐MYH is expressed in proliferating myoblasts but not in quiescent MuSCs, we reason that the extent of myoblast proliferation has decisive effects on the size of the quiescent MuSC pool.
Synopsis:
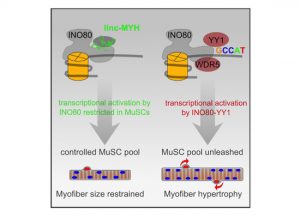
How chromatin remodeling complexes are tailored to specific cellular contexts remains unclear. Here, the conserved long non‐coding RNA linc‐MYH is shown to re‐configure the ubiquitous INO80 complex in proliferating murine skeletal muscle stem cells (MuSCs), establishing a functional unit to prevent non‐physiological myofiber growth.
- Linc‐MYH interacts with the INO80 complex in MuSCs, thereby limiting recruitment of YY1 and WDR5.
- Linc‐MYH selectively regulates transcriptional activation in trans but leaves INO80 DNA repair functions unaffected.
- Regulation of late‐stage expansion by linc‐MYH controls the number of quiescent MuSCs and muscle growth in vivo.