March 2020
J Clin Invest. doi: 10.1172, 2020
Microenvironmental Th9- and Th17- lymphocytes induce metastatic spreading in lung cancer
Salazar Y, Zheng X, Brunn D, Raifer H, Picard FS, Zhang Y, Winter H, Günther S, Weigert A, Weigmann B, Dumoutier L, Renauld JC, Waisman A, Schmall A, Tufman A, Fink L, Brüne B, Bopp T, Grimminger F, Seeger W, Pullamsetti SS, Huber M, Savai R.
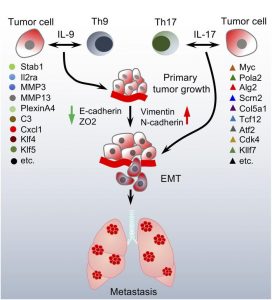
Immune microenvironment plays a critical role in lung cancer control versus progression and metastasis. In this investigation, we explored the impact of tumor-infiltrating-lymphocyte subpopulations on lung cancer biology by studying in vitro co-cultures, in vivo mouse models and human lung cancer tissue. Lymphocyte conditioned media-(CM) induced epithelial-mesenchymal-transition (EMT), and migration in both primary human lung cancer cells and cell lines. Correspondingly, major accumulation of Th9 and Th17 cells was detected in human lung cancer tissue, and correlated with poor survival. Co-culturing lung cancer cells with Th9/Th17 cells or exposing them to the respective-CM induced-EMT in cancer cells and modulated the expression profile of genes implicated in EMT and metastasis. These features were reproduced by the signatory cytokines IL–9 and IL–17, with gene regulatory profiles evoked by these cytokines partly overlapping and partly complementary. Co-injection of Th9 and/or Th17 cells with tumor cells in wildtype, Rag1-/-, Il9r-/- and Il17ra-/- mice altered tumor growth and metastasis. Accordingly, inhibition of IL–9 or IL–17 cytokines by neutralizing antibodies decreased EMT and slowed lung cancer progression and metastasis. In conclusion, Th9 and Th17 lymphocytes induce lung cancer cell EMT, thereby promoting migration, and metastatic spreading and offering for novel therapeutic strategies.