January 2021
Circulation
BMP9 and BMP10 Act Directly on Vascular Smooth Muscle Cells for Generation and Maintenance of the Contractile State
Abstract:
Background: Vascular smooth muscle cells (VSMCs) show a remarkable phenotypic plasticity allowing acquisition of contractile or synthetic states but critical information is missing about the physiological signals, promoting formation and maintenance of contractile VSMCs in vivo. BMP9 and BMP10 are known to regulate endothelial quiescence after secretion from the liver and right atrium, whereas a direct role in the regulation of VSMCs was not investigated. Here, we studied the role of BMP9 and BMP10 for controlling formation of contractile VSMCs.
Methods: We generated several cell type-specific loss- and gain-of-function transgenic mouse models to investigate the physiological role of BMP9, BMP10, ALK1 and SMAD7 in vivo. Morphometric assessments, expression analysis, blood pressure measurements, single molecule fluorescence in situ hybridization (FISH) were performed together with analysis of isolated pulmonary VSMCs to unravel phenotypic and transcriptomic changes in response to absence or presence of BMP9 and BMP10.
Results: Concomitant genetic inactivation of Bmp9 in the germ line and Bmp10 in the right atrium led to dramatic changes in vascular tone and diminution of the VSMC layer with attenuated contractility and decreased systemic as well as right ventricular systolic pressure (RVSP). Vice versa, overexpression of Bmp10 in endothelial cells (ECs) of adult mice dramatically enhanced formation of contractile VSMCs and increased systemic blood pressure as well as RVSP. Likewise, BMP9/10 treatment induced an ALK1-dependent phenotypic switch from synthetic to contractile in pulmonary VSMCs. SMC specific overexpression of Smad7 completely suppressed differentiation and proliferation of VSMCs and reiterated defects observed in adult Bmp9/10 double mutants. Deletion of Alk1 in VSMCs recapitulated the Bmp9/10 phenotype in pulmonary but not in aortic and coronary arteries. Bulk expression analysis and single molecule RNA-FISH uncovered vessel bed-specific, heterogeneous expression of BMP type 1 receptors, explaining phenotypic differences in different Alk1 mutant vessel beds.
Conclusions: Our study demonstrates that BMP9 and BMP10 act directly on VSMCs for induction and maintenance of their contractile state. Surprisingly, the effects of BMP9/10 in VSMCs are mediated by different combinations of BMP type 1 receptors in a vessel bed specific manner, offering new opportunities to manipulate blood pressure in the pulmonary circulation.
Model of the role of BMP9 and BMP10 for generation of contractile VSMCs in different vessels.
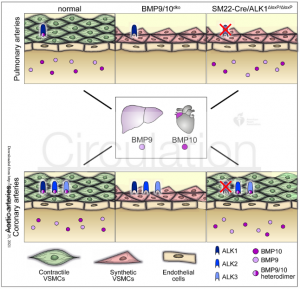
BMP9 and BMP10 are released into the blood stream by the liver (BMP9) and the right atrium (BMP10). BMP9 and BMP10 pass the endothelial barrier to directly stimulate formation of contractile VSMCs via different ALK receptors. Concomitant loss of Bmp9 and Bmp10 leads to a massive loss of contractile VSMCs in all muscularized vessels. Since pulmonary arteries express Alk1 but not Alk2 and Alk3 at high levels, inactivation of Alk1 in SMCs recapitulates the Bmp9/10 mutant phenotype only in pulmonary arteries but not aortic and coronary arteries, which also express Alk2 and Alk3, potentially compensating for the absence of
Alk1.