December 2020
Circulation
Mapping the Endothelial Cell S-Sulfhydrome Highlights the Crucial Role of Integrin Sulfhydration in Vascular Function
Sofia-Iris Bibli , Jiong Hu , Mario Looso , Andreas Weigert , Corina Ratiu , Janina Wittig , Maria Kyriaki Drekolia , Lukas Tombor , Voahanginirina Randriamboavonjy , Matthias S. Leisegang , Philipp Goymann , Fredy Delgado Lagos , Beate Fisslthaler , Sven Zukunft , Anastasia Kyselova , Alberto Fernando Oliveira Justo , Juliana Heidler , Diamantis Tsilimigras , Ralf P. Brandes , Stefanie Dimmeler , Andreas Papapetropoulos , Stefan Knapp , Stefan Offermanns , Ilka Wittig , Stephen L. Nishimura , Fragiska Sigala , and Ingrid Fleming (fleming(at)em.uni-frankfurt.de)
Abstract:
Background: In vascular endothelial cells, cysteine metabolism by the cystathionine γ lyase (CSE), generates hydrogen sulfide-related sulfane sulfur compounds (H2Sn), that exert their biological actions via cysteine S-sulfhydration of target proteins. This study set out to map the “S-sulfhydrome” i.e. the spectrum of proteins targeted by H2Sn in human endothelial cells.
Methods: LC-MS/MS was used to identify S-sulfhydrated cysteines in endothelial cell proteins and β3 integrin intra-protein disulfide bond rearrangement. Functional studies included endothelial cell adhesion, shear stress-induced cell alignment, blood pressure measurements and flow-induced vasodilatation in endothelial cell-specific CSE knock out mice and a small collective of patients with endothelial dysfunction.
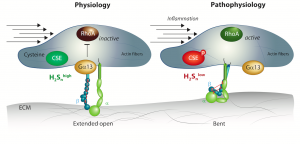
Results: Three paired sample sets were compared: (1) native human endothelial cells isolated from plaque-free mesenteric arteries (CSE activity high) and plaque-containing carotid arteries (CSE activity low), (2) cultured human endothelial cells kept under static conditions or exposed to fluid shear stress to decrease CSE expression, and (3) cultured endothelial cells exposed to shear stress to decrease CSE expression and treated with solvent or the slow-releasing H2Sn donor, SG1002. The endothelial cell “S-sulfhydrome” consisted of 3446 individual cysteine residues in 1591 proteins. The most altered family of proteins were the integrins and focusing on β3 integrin in detail we found that S-sulfhydration affected intra-protein disulfide bond formation and was required for the maintenance of an extended-open conformation of the β leg. β3 integrin S-sulfhydration was required for endothelial cell mechanotransduction in vitro as well as flow-induced dilatation in murine mesenteric arteries. In cultured cells, the loss of S-sulfhydration impaired interactions between β3 integrin and Gα13, resulting in the constitutive activation of RhoA and impaired flow-induced endothelial cell realignment. In humans with atherosclerosis, endothelial function correlated with low H2Sn generation, impaired flow-induced dilatation and a failure to detect β3 integrin S-sulfhydration, all of which were rescued following the administration of an H2S supplement.
Conclusions: Vascular disease is associated with marked changes in the S-sulfhydration of endothelial cell proteins involved in mediating responses to flow. Short term H2Snsupplementation improved vascular reactivity in humans highlighting the potential of interfering with this pathway to treat vascular disease.
Graphic Abstract: